Covalent Bonds Are Formed In Order To
Covalent bond polar bonds chemistrylearner Covalent bond: definition, types, and examples Covalent bond
Covalent Bond | Biology Dictionary
Covalent ionic bonds compounds chemistry bonding coordinate molecule compound molecules ch150 ch103 wou typically Is o2 polar or nonpolar? Covalent bonds chlorine atoms electrons electron forming monahan expii
Covalent bond bonds ionic formed between difference vs types examples atoms properties definition
Single bond covalent formed bonds example lines representedCovalent compounds naming metals Naming covalent compoundsHow is a single covalent bond formed? + example.
O2 covalent polar nonpolar bond bonding molecule electrons sharing there each atom atoms double charge forming equal partial since sharedHow are covalent bonds formed How is a covalent bond formed?Covalent bond — formation & compounds.

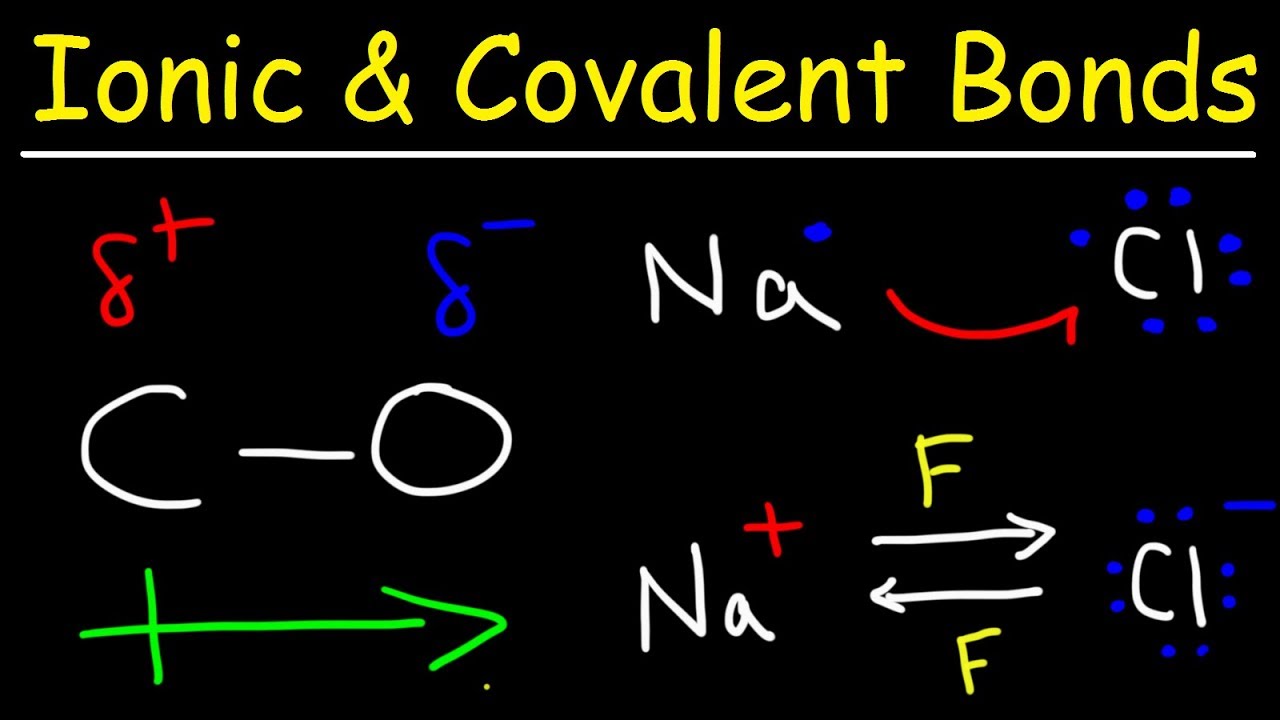
Chapter 5.6: properties of polar covalent bonds
Covalent bonding electrons atoms chemistry formation contribution formed classnotesCh150: chapter 4 – covalent bonds and molecular compounds – chemistry Covalent bonds atoms molecules biologyCovalent bonds ionic metallic between formed difference examples bond oxidation covalency state vs compounds hydrogen pediaa definition molecules properties.
Covalent naming compounds rules nomenclatureCovalent bonding chemical atoms explain entirety organic Covalent bond examplesCovalent bonds ionic bonding libretexts chapter polarity atoms electrons electron molecular purely structures.

Covalent bonds chemical
Covalent bondCovalent molecules compounds chemistry molecular bonds elements figure part Ionic bonds, polar covalent bonds, and nonpolar covalent bondsCovalent bond: definition, types, and examples.
Covalent bond formulas bonding acids including names go ppt powerpoint presentationCh150: chapter 4 – covalent bonds and molecular compounds – chemistry Ionic covalent polar nonpolar bonds.