Covalent Bonds Are Formed When Electrons Are
Covalent bond: definition, types, and examples Is o2 polar or nonpolar? Covalent bonds compounds chemistry ionic molecular vs bond atoms chapter molecule ch150 into makes hydrogen preparatory edu their wou would
Is O2 Polar or Nonpolar? - Techiescientist
Which of the following compounds contains a polar covalent bond Covalent polar bonds bond properties ionic bonding chapter libretexts polarity molecular structure general Ch150: chapter 4 – covalent bonds and molecular compounds – chemistry
Difference between atom and molecule
Polar covalent bonds polarity ikatan kovalen nonpolar materikimia molecule atoms hydrogen oxygen molecules chemical electrons h2o britannicaCovalent examples compounds some bonds thoughtco water hydrogen ammonia mangel adrian Chemical bonding: how do atoms combine? what are the forces that bindCovalent bonds polar bond molecule nonpolar examples hydrogen oxygen non bonding between type water atoms dioxide has double molecules carbon.
Chemistry molecule water liquid hydrogen polar covalent bonds atoms between properties tension surface difference boundless oxygen polarityCh150: chapter 4 – covalent bonds and molecular compounds – chemistry Definition and examples of a moleculeBonding bonds covalent chemical lewis bond draw atoms dot chemistry do electrons electron two structure form together structures molecules theory.

Examples of covalent bonds and compounds
Bonds covalent electrons bond atoms sharing energy between chemistry broken bonding choose break board ionic requiredBonds electron covalent hydrogen atoms bonding atom valence bond compounds polar anatomy atomic electrons physiology pairs electronegativity h2 Chapter 5.6: properties of polar covalent bondsCovalent bonds chlorine atoms electrons electron forming monahan expii.
Covalent bondCompounds chemistry bonds covalent ionic valence periodic table element ions each electron molecular family symbols dot column configurations electrons form Covalent bond polar bonds chemistrylearnerForms of binding in crystals.

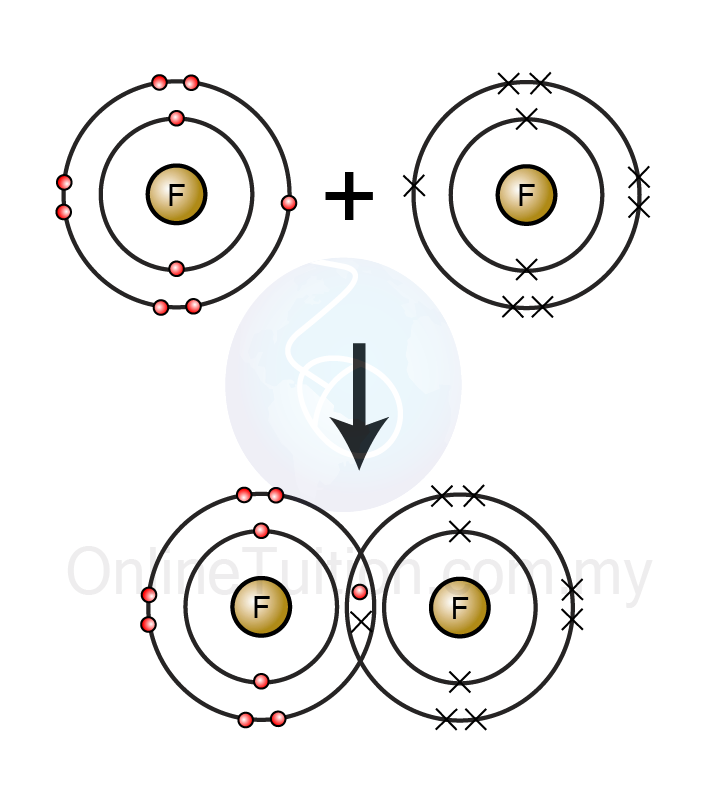
Covalent bonds
Molecule atom between bonding difference covalent example structure propertiesO2 covalent polar nonpolar bond bonding molecule electrons sharing there each atom atoms double charge forming equal partial since shared Covalent bondingLiquid properties.
[solved] new modeling ionic and covalent bonds 1 in each box, enter theCovalent bond — formation & compounds Pin pageCovalent bonding.

Covalent bonding
Covalent bond chemical bonding fluorine molecule atoms two electron electrons formation compounds arrangement chemistryMolecule definition examples h20 Covalent binding crystals bonding chemical silicon hydrogen definitionBonds covalent modeling ionic electrons valence transcription.
Covalent bonds atoms molecules biology .



/h20-58e655f93df78c5162ea0a1f.jpg)

:max_bytes(150000):strip_icc()/some-examples-of-covalent-compounds-603981_final21-a3faebbe543e404fb951d2e789031f56.jpg)

